- Overload of the patellofemoral joint, a condition known as “runner’s knee.”
- Disorders in the alignment of the patellofemoral joint with instability, commonly seen in young women.
- Enthesopathy or patellar tendinopathy, also known as “jumper’s knee.” In children, this condition presents as patellar osteochondrosis.
“Jumper’s knee” (JK) is a very common condition among athletes and one of the most frequent causes of anterior knee pain in both children and adults, particularly those who participate in sports that involve jumping.
It is considered a tendinopathy of the patellar tendon, producing pain in the anterior region of the knee, specifically at the lower pole of the patella. This condition results from overuse of the knee extensor mechanism combined with repetitive mechanical stress. It is associated with sports activities involving jumping, landing, acceleration, and deceleration. For this reason, it is commonly seen in athletes who play volleyball, basketball, participate in long and high jump, or engage in long-distance running.
However, despite its high prevalence and the existence of many research studies, a definitive causal explanation has not been reliably established. When considering the anatomy of the tendon at its bony insertion, the condition actually corresponds to an “enthesopathy,” or a disorder of the enthesis organ (the transitional zone where tendon tissue connects to bone).
Anatomy of the Knee Extensor Mechanism (figs 1 and 2)
-
Quadriceps (Q): composed of four muscles:
- Rectus femoris: located anteriorly, extending from the anterior inferior iliac spine to the upper pole of the patella; it is biarticular.
- Vastus lateralis, intermedius (cruralis), and medialis: these insert proximally on the femoral shaft and distally on the patella via their tendon. The vastus medialis is the primary stabilizer of the patella.
-
Quadriceps tendon (QT): formed by the convergence of the four quadriceps muscles. It inserts into the upper pole of the patella in three layers:
- Superficial layer: formed by the rectus femoris, located anteriorly.
- Intermediate layer: formed by the vastus lateralis and vastus medialis.
- Deep layer: formed by the vastus intermedius (cruralis).
- Reflected tendon: a very thin, ribbon-shaped tendon that continues from the rectus femoris tendon. It passes superficially over the patella and reaches the lower pole, forming the patellar tendon, which inserts into the anterior tibial tuberosity.
- Patella (P): the patella is the convergence point of the quadriceps muscle tendons at its upper pole. The patellar tendon originates at its lower pole. Its function is to act as a fulcrum for the extensor mechanism, increasing its leverage and power.
- Retinacula: although the patellar retinacula are considered part of the extensor mechanism due to their relation to the vastus muscles, injury to them does not affect its function.
- Patellar tendon (PT): an extension of the reflected tendon, running from the lower pole of the patella to the anterior tibial tuberosity, where it inserts.
- Anterior tibial tuberosity (ATT): a bony prominence located on the anterior aspect of the proximal tibial metaphysis, serving as the distal insertion point of the extensor mechanism via the patellar tendon.
Figure 2. Diagram of the extensor mechanism, lateral view. Q – Quadriceps, QT – Quadriceps tendon, P – Patella, PT – Patellar tendon, ATT – Anterior tibial tuberosity. Source: ScienceDirect.
The Enthesis
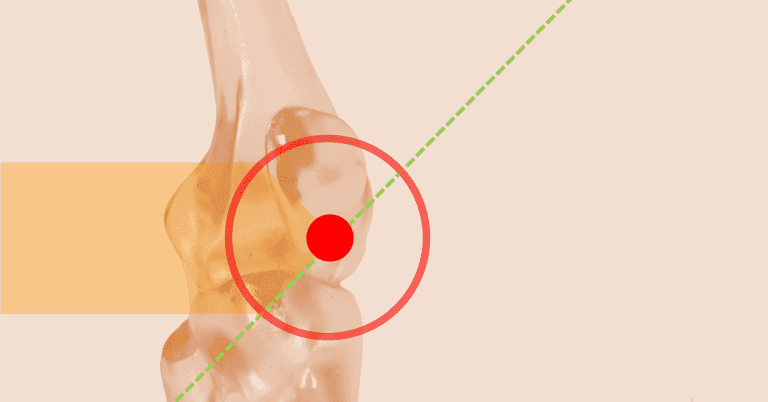
The enthesis is the region where a tendon, ligament, joint capsule, or muscle fascia attaches to the bone. It is a transitional tissue that serves as an anchor for soft tissues and transfers stress from these areas to the adjacent bone. (fig 3)
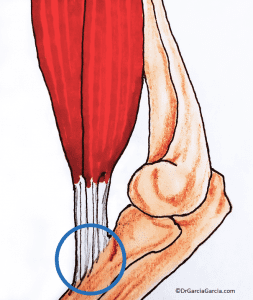
Enthesopathy is a term that indicates the presence of pathology in the enthesis, regardless of its etiology. (fig 4)
Figure 3. Enthesis. The circled area shows the junction between the tendon and bone, referred to as the “enthesis.”
Figure 4. Close-up illustration of the tendon-bone anchor. Source: Fisioterapia Senso.
Etiology
Among the theories regarding the etiology of JK (mechanical, vascular, or impingement-related causes), the most widely accepted is the mechanical cause, due to repetitive overload of the tendon. According to this theory, overload leads to progressive weakening of the tissue and, eventually, its rupture.
The area with the greatest increase in tension is the deep posterior portion of the tendon, closer to the knee’s center of rotation, located at the inferior pole of the patella. Tension increases with knee flexion, which is why it is strongly associated with excessive flexion during weighted squats and landing after a jump.
The Squat (figs 5 and 6)
The squat is performed by flexing the hips and knees while keeping the trunk upright with slight lumbar extension. The hips undergo hyperflexion; however, the knees should be flexed so that the thighs remain parallel to the ground (at 90°). The primary cause of knee injury is generally poor technique, particularly when lifting weights in a hyperflexed position (“deep squat,” with the knees flexed beyond 90°).
The Jump (fig 7)
During jumping, the mechanical moment that causes the greatest stress on the knee extensor mechanism—and is considered the most plausible cause of JK—is the “landing” or final phase of the jump. (3)
The landing phase involves synchronized movements of trunk flexion, hip and knee flexion, and ankle dorsiflexion. This combination helps absorb the impact through the muscles, reducing stress on the bones and joints, and resulting in a “soft” (almost silent) landing. In contrast, landing with the trunk, hips, and knees extended and without ankle dorsiflexion is very stiff or “hard,” causing an audible impact with the ground and increasing osteoarticular load, thereby raising the risk of injury.
During jumping, the knee plays a crucial role in load transfer. The load is transmitted through the patellar tendon, dissipating energy and protecting the joints of the lower limbs. The most frequently affected area is the inferior pole of the patella (origin of the patellar tendon), but it may also involve the insertion of the quadriceps tendon at the superior patellar pole or the insertion of the patellar tendon at the tibial tuberosity.
Figure 5. Squat, front view. Foot positioning aligns with shoulder width.
Figure 6. Squat, lateral view. In a full squat, the thighs are parallel to the floor. Greater flexion (deep squat) increases the risk of extensor mechanism injury.
Figure 7. Jump. a) Takeoff, b) and c) Landing. Synchronous flexion of the hip and knee joints, along with ankle dorsiflexion, enables a soft landing with minimal injury risk.
Pathophysiology of Enthesopathy
The lesion is located at the tendon-to-bone insertion site (enthesis), making it an “enthesopathy” rather than a tendinopathy. It is not considered inflammatory but rather degenerative due to the presence of “angiofibroblastic” tissue (formed by neovascularization) at the lesion site, accompanied by microtears in the tendon tissue, hyaline degeneration, and metaplasia, resulting in what is known as “mucoid degeneration.” Neovascularization has been shown to contribute to tendon degeneration and rupture.
Several other factors are involved in the development of enthesopathy, including body mass index (BMI), leg length discrepancy, height of the foot’s plantar arch, quadriceps strength and flexibility, hamstring flexibility, and vertical jump height. (4)
Classification
Classification
Two classification systems are used to describe and assess the severity of the condition:
-
Blazina Classification: Based on severity. (1,5)
- Grade 1: Pain occurs after sports activity.
- Grade 2: Pain at the beginning of sports activity that disappears with warm-up and occasionally returns with fatigue.
- Grade 3: Pain at rest and during activity, along with decreased performance.
- Grade 4: Tendon rupture.
-
Kaux Classification: Based on duration of symptoms. (2)
- Acute phase: Symptoms present for 0 to 6 weeks.
- Subacute phase: Symptoms present for 6 to 12 weeks.
- Chronic phase: Symptoms present for more than 3 months.
Clinical Presentation (1)
There is typically a history of activities involving jumping; the patient complains of anterior knee pain. Sudden pain occurs under load and disappears when the load is removed; it reappears when sitting, squatting, climbing stairs, or even after sitting for long periods with the knee in deep flexion (known as the “theater sign”). Pain rarely occurs at rest.
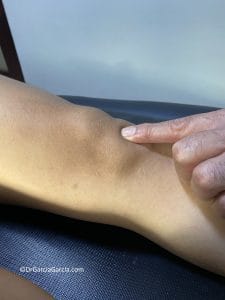
The painful area is located at the inferior pole of the patella. Examination is performed with the patient in the supine position and the knee extended, palpating and pressing the patella against the femur and checking for pain during passive movement of the femoropatellar (patellofemoral) joint. This movement should be painless and without crepitus.
Provoked pain decreases when the same pressure is applied with the knee flexed at 90° (Basset’s sign); this occurs because pain at the inferior pole of the patella is reduced when the quadriceps is contracted.
If palpation is performed with the patient standing—first with the knee extended and then flexed at 30°—the pain is less intense in the latter position (active quadriceps sign in standing position).
Figure 8. a) During knee examination, pain appears at the inferior pole of the patella when pressure is applied (arrow). b) The painful area becomes more apparent when the inferior pole is manually lifted by pressing the patella against the femur from proximal to distal.
Diagnostic Methods
In general, clinical evaluation is sufficient for an accurate diagnosis; however, imaging studies can be helpful.
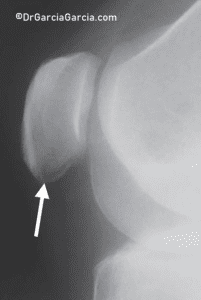
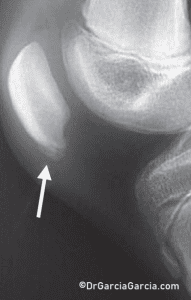
X-rays may reveal changes in the tendon, such as elongation of the inferior pole of the patella, calcification, inferior traction spur (enthesophyte), and increased density within the matrix of the patellar tendon. (fig 9)
Ultrasound and magnetic resonance imaging (MRI) can detect abnormalities in the patellar tendon and enthesis, and may show thickening of the patellar tendon or the presence of edema with areas of increased signal intensity.
Figure 9. Lateral knee X-ray in a case of inferior patellar pole pain. A transverse radiolucent line resembling an avulsion fracture is frequently observed.
Differential Diagnosis
In children, jumper’s knee is equivalent to the “Sinding-Larsen-Johansson syndrome,” which causes similar symptoms and has similar treatment approaches. (fig 10)
Figure 10. X-ray of a child’s knee with pain at the inferior pole of the patella. This corresponds to an osteochondrosis due to Sinding-Larsen-Johansson disease.
Treatment
In the early stages of the condition, most cases of jumper’s knee (JK) resolve with medical and rehabilitation treatment. However, in other cases, treatment outcomes are unsatisfactory, resulting in prolonged periods without sports participation and a high recurrence rate (over 25%). Up to 50% of athletes with JK end their sports careers due to recurrence or lack of response to various treatments.
Conservative Treatment
- Anti-inflammatory medication: NSAIDs are frequently used and have shown short-term benefits. However, their use has been questioned, as chronic lesion histopathology shows no presence of inflammatory cells.
- Relative rest: Total immobilization is not recommended as it can lead to tendon and muscle atrophy.
- Cryotherapy: This is highly effective in providing analgesia and apparently reducing neovascularization. However, it should not be used before physical activity, as it may mask pain and lead to increased severity of injury due to overloading.
- Modification of activities and sports training: Includes proper warm-up and physiotherapy to improve quadriceps and hamstring flexibility. Eccentric exercises are recommended due to their superior results. Eccentric training is considered the treatment of choice for patients with JK, as it has demonstrated outcomes as effective as surgical treatment and is superior to concentric exercises. This approach can be implemented over twelve weeks as a trial before considering surgical intervention. Opinions vary regarding the duration, frequency, and type of exercise: drop squats, slow eccentric movements, or using a slanted board.
- Extracorporeal shock wave therapy: Shock waves applied to the injured tendon generate significant mechanical forces and may offer analgesic benefits by breaking down calcium deposits and stimulating tissue repair. There is no consensus on application method, type of wave generation, energy level, location, treatment frequency, or use of anesthesia.
- Infrapatellar strap: Used to reduce tension on the patellar tendon by altering the angle between the patella and the tendon.
Other treatment alternatives include hyperthermia-based thermotherapy and dry needling.
Due to the refractory response to many initial treatments, new local treatment methods have recently emerged, including patches and injections.
- Application of glyceryl trinitrate patches: Theoretical action involves delivering nitric oxide to the pathological tendon to stimulate fibroblast proliferation and collagen synthesis.
-
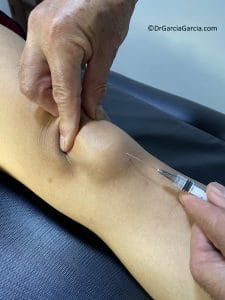
Injections at the lesion site: (fig 11)
- Sclerosing agent (prolotherapy): A chemical irritant (such as etoxisclerol) is injected to promote an inflammatory response and restructuring of the mucoid area. Carries a risk of anaphylaxis.
- Corticosteroids: Frequently used and provide short-term pain relief, but they increase the risk of tendon rupture. Theoretically, based on their mechanism of action, they are not indicated.
- Platelet-rich plasma (PRP): PRP is also widely used and has shown good outcomes, although there is no evidence-based indication for its use. (6)
- Aprotinin: Reported to yield better results than steroids and with a low risk of anaphylaxis even with repeated use. Aprotinin is a strong inhibitor of matrix metalloproteinases and is part of “fibrin glue” used to promote healing and reduce intraoperative blood loss. (7)
Regardless of the type of treatment, patients should avoid activities such as excessive jumping or high-impact knee loading, as these only exacerbate the condition.
Figure 11. Injection directly into the inferior pole of the patella, at the enthesis site, with manual elevation by pressing the patella against the femur from proximal to distal.
Surgical Treatment
Surgical intervention is indicated in cases of partial tendon tears and Blazina stage III, where pain persists during rest and activity, affecting performance. It remains the last resort for chronic, refractory cases that do not respond to conservative treatment.
Surgical Treatment
The surgical procedure consists of open debridement of the inferior pole of the patella and the patellar tendon by removing angiofibroblastic and degenerative tissue, followed by tendon reinsertion using sutures or anchors. It may be accompanied by additional procedures such as:
- Drilling of the patellar pole.
- Partial resection of the patellar pole.
- Resection of hypertrophic synovium and fat pad.
Prognosis
In general, jumper’s knee is resolved with conservative management. However, the return to sports is usually slow. The timeline depends on several factors, including the severity of pain, degree of dysfunction, the sport practiced, and the quality of rehabilitation.
In mild cases, the patient typically returns to sports within about 20 days. In severe cases, it may take around three months, sometimes requiring 6 to 12 months. Additionally, mild to moderate pain may persist for many years, though it does not usually limit recreational physical activity.
Conclusions
- Jumper’s knee is specifically a degenerative enthesopathy, not an inflammatory condition.
- The most widely accepted theory regarding its origin is mechanical overload of the knee extensor mechanism at the inferior pole of the patella.
- Patients may experience fear when informed of the increased risk of tendon rupture, which can negatively affect functional outcomes.
- Overreliance on various therapies without incorporating rehabilitation exercises—particularly eccentric exercises, which are the foundation of treatment—often leads to poor results.
- Jump landing techniques should be evaluated and corrected, and athletes should retrain landing mechanics after appropriate rehabilitation.
- There is limited support for recommending reduced landing stiffness by improving ankle dorsiflexion and trunk flexion range to achieve a softer landing pattern.
- Cryotherapy should not be used prior to competition; it can impair motor function, mask pain, and increase the risk of reinjury.
- Intralesional platelet-rich plasma (PRP) has shown good results, but evidence-based recommendations on its effectiveness cannot yet be made.
- Surgical intervention has provided good to excellent results in the majority of patients.
- Athletes, physicians, and athletic trainers recognize that treating jumper’s knee can be a slow, challenging, and at times frustrating process.
Bibliography
- Schwartz A, Watson JN, Hutchinson MR. Patellar Tendinopathy. Sports Health. 2015 Sep-Oct;7(5):415-20. PMID: 26502416
- Santana JA, Mabrouk A, Sherman Al. Jumper’s Knee. [Updated 2022 Feb 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–
- Tayfur A, Haque A, et al. Are Landing Patterns in Jumping Athletes Associated with Patellar Tendinopathy? A Systematic Review with Evidence Gap Map and Meta-analysis. Sports Med. 2022 Jan; 52(1):123-137. PMID: 34554424
- Worp H, Ark M, Roerink S, Pepping GJ, Akker-Scheek I, Zwerver J. Risk factors for patellar tendinopathy: a systematic review of the literature. Br J Sports Med. 2011; 45:446-452.
- Blazina ME, Kerlan RK, et al. Jumper’s Knee. Orthop Clin North Am. 1973; 4:665–78.
- Gosens T, Den Oudsten BL, et al. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: a prospective cohort study and the influence of previous treatments. Int Orthop. 2012 Sep;36(9):1941-6. PMID: 22534958.
- Orchard J, Massey A, Brown R, Cardon-Dunbar A, Hofmann J. Successful management of tendinopathy with injections of the MMP-inhibitor aprotinin. Clin Orthop Relat Res. 2008 Jul;466(7):1625-32. PMID: 18449616.
What would you like to share with Dr. García? Your feedback is valuable to us. Please share your thoughts!
Discover more from Dr. Roberto García
Subscribe to get the latest posts sent to your email.